Authors
- Cancer Registry Process Mapping Task Force, a task force of the Canadian Council of Cancer Registries
Most PTCR information collected is from provincial/territorial level health information systems that are attributed to universal health care systems in Canada.
Canadian cancer surveillance consists of 13 provincial and territorial population-based cancer registries (PTCR) that collect information on all reportable tumors among Canadian residents according to Canadian and international coding standards. Although PTCR practice may differ across the provinces, most of the information collected is ascertained from provincial/territorial level health information systems attributed to universal health care systems in Canada 1.
Canadian cancer registries typically report over 90% of tumors as pathology confirmed. To facilitate data quality, timeliness, and complete ascertainment of pathology-confirmed cases, improvements in electronic reporting from pathology laboratories continue in many jurisdictions in Canada and the United States.
Addressing Challenges
Some PTCRs rely on manual processes, including but not limited to filtering electronic reports for reportability status, storing and processing massive volumes of data, and maintaining these systems. Although some advances have been made, we are far from fully realizing the benefits of real-time electronic reporting of cancer pathology. NAACCR, along with cancer registry experts and students from Rutgers University, recently published work in this area titled: A Six Sigma Lean Green Belt Analysis of Electronic Pathology Reporting in Central Cancer Registries. 2
Canadian cancer registries are reporting common experiences. Data volume and complexity of data are increasing, human resource capacity is limited, and there is increased pressure to improve on data timeliness. In response to these challenges, and in light of advances in electronic pathology reporting, the Canadian Council of Cancer Registries (CCCR) created a task force (Cancer Registry Data Process Mapping Task Force) to lead an environmental scan of electronic reporting of pathology information across PTCRs. This effort was designed to facilitate additional discussions among PTCRs, not necessarily providing a written compendium of solutions.
The overarching objectives of this work were to:
- Enable comparison of pathology data sources, systems, processes and tools across PTCRs
- Facilitate the capacity to:
- Leverage systems, processes, tools and knowledge from other PTCRs
- Identify and compare strengths, weaknesses and pain points
- Identify opportunities for improvements, enhance collaboration, and share advancements
- Prove the opportunity to identify challenges in ascertaining, integrating and consolidating pathology information, and contribute towards Pan-Canadian solutions, registry practices and data standards.
Engagement With PTCRs
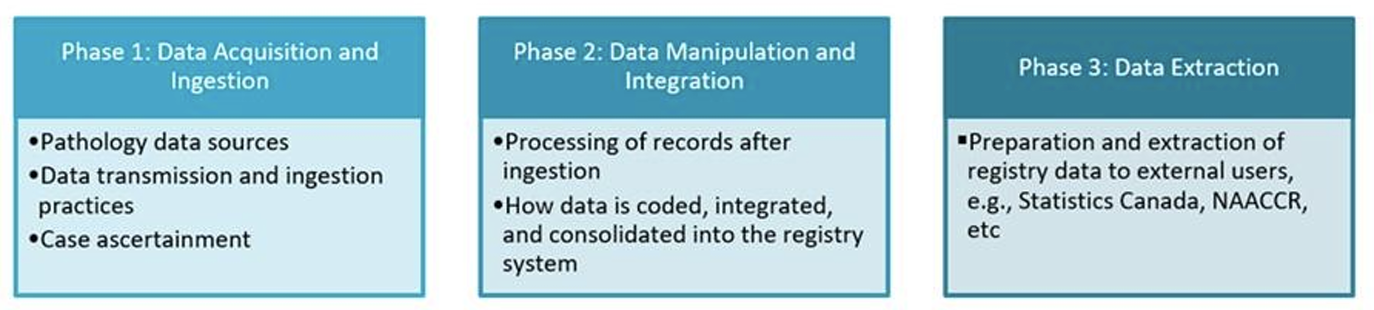
The environmental scan is currently underway and will be conducted in three phases, corresponding to the different stages of cancer registry operations. Under each phase, a questionnaire was developed by the Task Force in collaboration with cancer registry experts across Canada to gather key information about electronic reporting of pathology. The phases and topic areas were as follows:
In partnership with Statistics Canada, follow up interviews were held with PTCR registry teams to discuss responses to the questionnaire and develop key project outputs:
- Data metrics table
- Cancer registry data process maps
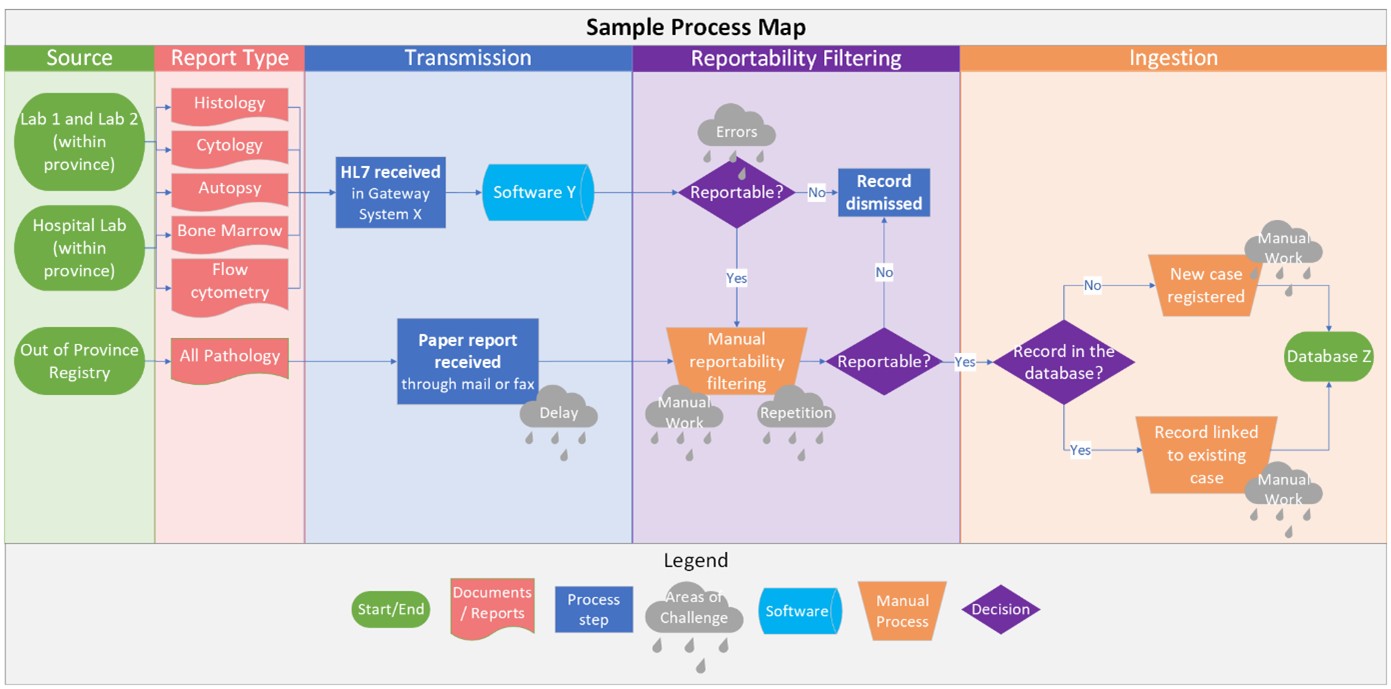
The data metrics table compares high-level information on systems, processes, and data that characterize electronic reporting of pathology across PTCRs. The cancer registry process maps (i.e., flow diagrams as shown below) incorporate information on systems and processes, areas that rely on manual work, pain points, challenges, and opportunities.
Early Findings and Key Messages
To date, the Task Force in partnership with Statistics Canada, completed Phase 1, and was able to engage nearly all PTCRs. Cancer registry process maps illustrating where we rely on manual work, common challenges, and opportunities, were shared with all PTCRs through the CCCR Web Portal. Additionally, a data metrics table was produced summarizing electronic reporting across all Canadian cancer registries. At this time, we are excited to share early findings of our work:
- Many PTCRs are in early phases of transitioning or recently transitioned to electronic reporting. In most cases, electronic reporting includes complete coverage of pathology information in the province.
- Even though all registries have established electronic reporting, the way those reports are fed into the system, the format of the reports, and technical solutions to process the information are significantly different.
- Many PTCRs are looking to explore data science in order to deal with the demands associated with volumes, timeliness, and human resource (registrar) shortages, but this is not traditionally an area within the cancer registries.
- Some registries are actively collaborating with data science teams in using natural language processing and machine learning to support operations.
The current work provides some early examples of successes, but the exercise was designed as proof of concept work; it provides limited direction on standards and methods for registrar practice. The second phase of this initiative is underway and will focus on the processing, consolidation, and integration of pathology data into the cancer registry dataset. We want to acknowledge the great work done by our NAACCR and US colleagues in this space as reported in the A Six Sigma Lean Green Belt Analysis of Electronic Pathology Reporting in Central Cancer Registries [2]. We are looking forward to engaging with US partners to explore new opportunities that arise from electronic reporting and harnessing the power of data science to modernize cancer registry practice.
REFERENCES:
- NAACCR Editorial Review Board. Central Cancer Registries in Canada: Strong Integration with Canadian Health Information Systems and a Single Standard Setter. NAACCR Narrative ↩
- North American Association of Central Cancer Registries. A Six Sigma Lean Green Belt Analysis of Electronic Pathology Reporting in Central Cancer Registries. ↩
Discover the different ways of mapping cancer registry processes to collect and process cancer pathology data in Canada.
What to Read Next
NAACCR December 2023 Call for Data Update
NAACCR has completed the data quality assessments of the December 2023 Call for Data (CFD) Submission File. We will be…
CiNA Writing Network Workgroup Seeks Members!
The CiNA Writing Network is a new Research & Data Use workgroup! This workgroup will oversee methods and research papers…
Looking Ahead: Changes Approved for 2025 Registry Data Standards
The High-Level Strategic Group (HLSG) voted earlier this month to approve changes to the NAACCR Data Standards and Data Dictionary…