Assessing the Completeness of Key Data Items Across NCCR Registries for Pediatric and Adult Cancer Cases

The National Childhood Cancer Registry (NCCR) was developed under the National Cancer Institute’s Childhood Cancer Data Initiative (CCDI) to identify and follow childhood cancer cases in the U.S. Its primary goal is to provide a platform to better understand the causes, outcomes, effective treatments, and late effects of cancer among children, adolescents, and young adults in the U.S.
There are three NCCR active working groups coordinated by the North American Association of Central Cancer Registries (NAACCR) to discuss various aspects involving utilization, patient confidentiality and protections, and other key components of the establishment and dissemination of the National Childhood Cancer Registry.
The NCCR Data Quality Working Group (DQ WG) aims to develop appropriate methods to monitor the quality and consistency of pediatric cancer data. One of its tasks is to check completeness of specific data items and investigate benchmarks for pediatric data item completeness.
Key data items submitted in the NCCR Call for Data include histology, primary site, and Summary Stage. The first two are needed to derive the International Classification of Childhood Cancer (ICCC) category, another key pediatric cancer data item. To assess the completeness of the selected data items in NCCR participating registries, we used the Median/Multiple Outlier Testing method (MMOT), a quality control tool developed by the NCI’s Surveillance Research Program to assess and monitor the quality of data submitted by SEER registries.
Methods
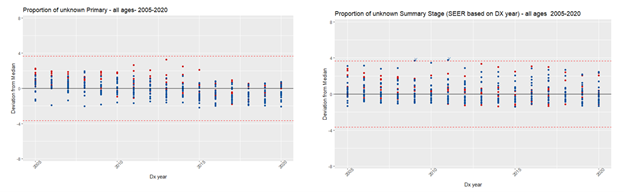
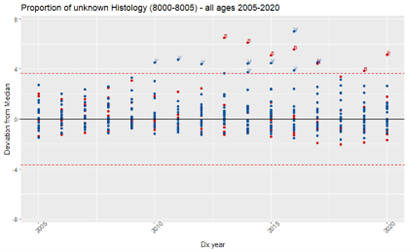
We used MMOT to identify outliers in the proportion of unknown values for the following data items: histology (unknown: 8000-8005), primary site (unknown: C76.0-C76.5, C76.7-C76.8, C80.9), Summary Stage (unknown: code 9), and ICCC (Ie, IIe, IIIf, VIc, VIIc, VIIIe, Ixe, Xe, XIf11, XIIb, not classified) by NCCR participating registry, in each year of the period 2005-2020, for all ages and by age groups (0-19; 20-39; and 40+). It should be noted that outliers don’t necessarily mean a registry is under-performing.
Results and Discussion
Results confirmed that, when checking completeness for all ages for primary site, and summary stage, we might be obscuring high proportions of unknown values for cases under 20 years old (Figure 1). However, for histology, the opposite occurred (Figure 2). The proportion of unknown histology was much lower for the age group 0-19 (Figure 3). The likely reason for this result is that, different from adult tumors, the diagnosis of childhood cancers prioritizes tumor morphology over primary site.
Another important result is that the proportion of unknown values was not related to the type of funding or registry (NPCR or SEER); it was only related to the age. These findings support the need for additional tools to assist registries in improving the quality of pediatric data.
The NCCR DQ WG will continue to evaluate completeness of additional key data items: grade, vital status, cause of death, brain molecular markers, and more. The NCCR team plans to work with NCCR registries to better understand the root cause of outliers.
Co-authors: Gonçalo Forjaz, DVM, MSc, ODS-C; Stephanie Hill, MPH, ODS-C; Betsy Kohler, MPH, ODS-C.
Tags: Childhood Cancer, Completeness, Featured, NCCR
What to Read Next
Advancing Global Collaboration in Childhood Cancer Research: Data Resource Mapping and Federated Network Matrix
In November 2023, the US National Cancer Institute (NCI) (with support from NAACCR) and the French National Cancer Institute (INCa)…
An Interview with Our New RDU Chair
An Interview with our new Research and Date Use Committee Chair It was so much fun interviewing the RDU Co-Chairs…