The Mid-Level Tactical Group (MLTG) is a collaborative body of cancer registry standard setters led by representatives from CDC NPCR and NCI SEER and coordinated by NAACCR.
Other member organizations include the Commission on Cancer (CoC), American Joint Committee on Cancer (AJCC), College of American Pathologists (CAP), the Canadian Council on Cancer Registries (CCCR) and the National Cancer Registrars Association (NCRA).
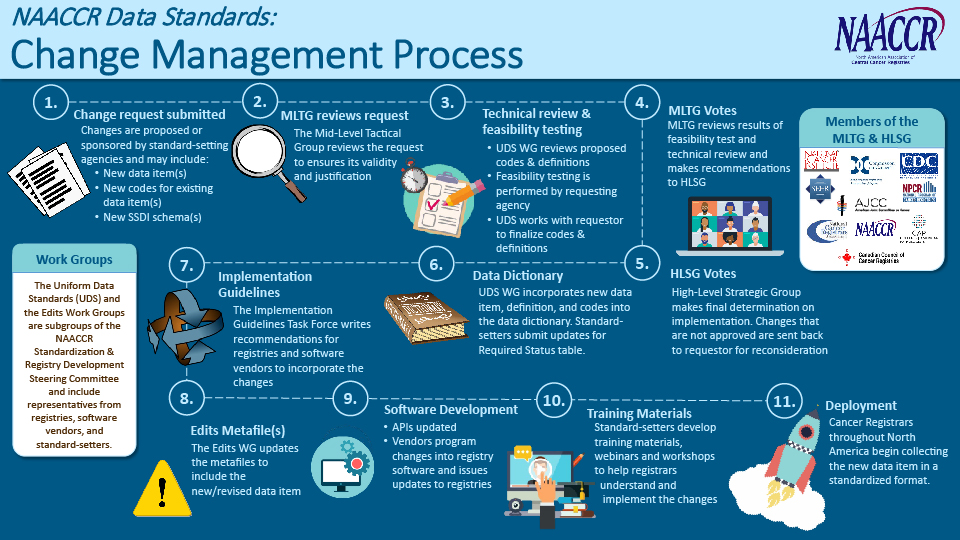
The primary purpose of the MLTG is to review requests for changes to the common NAACCR Data Standards and Data Dictionary (DS&DD). The NAACCR DS&DD includes most of the data items collected by hospital and central registries, although individual standard setters may choose to collect data items outside the NAACCR DS&DD.
The MLTG considers many factors when reviewing change requests, including scientific and clinical rationale, burden on registrars, availability of information in the medical record, technical feasibility, and field test results. Based on their review and with input from the Uniform Data Standards Work Group, the MLTG makes recommendations to the High-Level Strategic Group (HLSG), who has the ultimate authority to decide implementation.
Between June and October 2022, the MLTG received and reviewed 55 change requests for implementation in 2024: 28 requests for new data items and 26 requests for revisions to existing data items. After careful consideration and deliberation, including a special in-person session, the HLSG approved 23 of the proposed changes for implementation in 2024. The approved changes included six new data items (three derived and three manually coded), 12 revisions to existing data items, and retirement of five data items.
Summary of v24 Changes
New Data Items
- Rx Hosp–Recon Breast [751] & RX Summ–Recon Breast [1335]
- Brain Primary Tumor Location [3964]
- Derived Summary Grade 2018 [1975] (derived at central registry)
- Geocoding Quality Code [86] (derived at central registry)
- Geocoding Quality Code Detail [87] (derived at central registry)
Revised Data Items
- IHS PRCDA [194] – minor wording changes
- UHIO [284] – name change and minor wording changes
- UHIO City [285] – name change and minor wording changes
- Tobacco use smoking status [344] – minor wording changes
- EDP MDE Link Date [530] – minor wording changes
- EDP MDE Link [531] – minor wording changes
- RX Hosp-Surg Prim Site 2023 [671] & RX Summ-Surg Prim Site 2023 [1291] – new surgery codes for breast, colon, lung, pancreas, and thyroid
- SSDI Brain Molecular Markers [3816] – added codes and terminology
- P16 [#3956] – added vulva schema to SSDI
- Location of Radiation [15550] – minor wording changes
- SEER SSF#1 [#3700] – changed to 2 digits and added codes
Retired Data Items
- Birthplace [#250]
- Place of Death [#1940]
- CRC Checksum [#2081]
- Maiden Name [#2390]
- LN Status Femoral-Inguinal, Para-aortic, Pelvic [#3884]
Dig into the MLTG Change Control and Implementation Process
View the full-size change management process illustration.
The NAACCR Implementation Guidelines and Recommendations provide cancer registries and software vendors with a plan to assist with the implementation of the NAACCR Data Standards and Data Dictionary in a timely manner.
Tags: DS&DD, Featured, Mid-Level Tactical Group, Summer 2023
What to Read Next
Sharing NAACCR Pediatric Cancer Initiatives on Dr. Rick Greene’s Cancer Registry World Podcast
I am excited to share that I was recently interviewed by Dr. Rick Greene for his Cancer Registry World podcast….
The Importance of Professional Certification for Cancer Registrars
Professional certification plays a vital role in strengthening the cancer registry workforce and ensuring the high-quality data that is essential…
Nominations Opening Soon for the 2026-2027 Board of Directors
The Nominating Committee is seeking nominees to run for election to three key leadership roles on the NAACCR Board of…